Breadcrumb navigation


Our Approach
Our approach represents a unique convergence of science and technology, seamlessly integrating best-in-class AI and machine learning (ML) models with a range of delivery modalities, including viral vectors, bacterial vectors, and mRNA.
In-Silico Methods
Our in-silico methods for immuno-oncology and infectious disease vaccine development take an in-depth approach that models both T-cell and B-cell machinery to ensure a robust vaccine design.
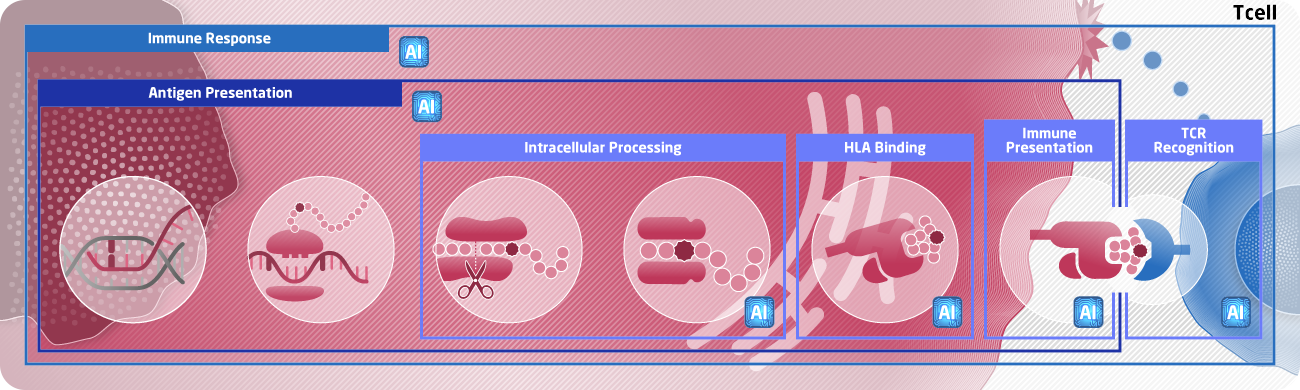
NEC Neoantigen Prediction Pipeline
NEC Bio has developed an AI engine, known as the NEC Immune Profiler, to predict true neoantigens from next generation sequencing data. These antigens are used for personalized cancer immunotherapy and cancer immunotherapy biomarkers. The key differentiator of our technology is the accurate prediction of antigens that are naturally processed and present on the tumor cell surface. This unique approach has been validated against clinically relevant neoantigens, increasing the probability of clinical success for our biopharma partners.
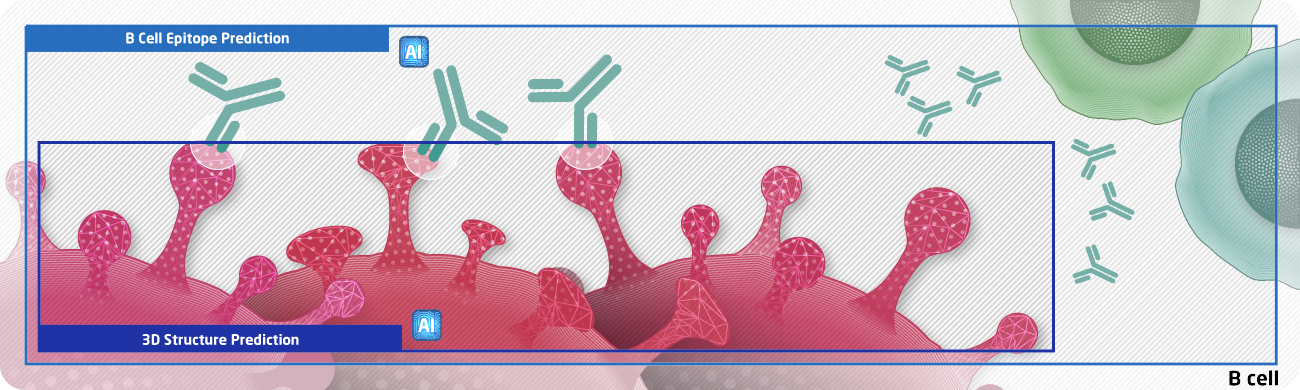
NEC Infectious Disease Tool Kit
We combine the antigen presentation to the infected-host cell surface and immunogenicity predictions of the NEC Immune Profiler with a robust Monte Carlo and digital twin simulation to profile the entire proteome of a target pathogen and identify a subset of epitope hotspots that could be harnessed in a vaccine formulation to provide a broad coverage across the population. Also, our tool kit contains the B-cell epitope predictor which replaces the antigen for antibody production and detection.

Vaccine Platforms
NECVAX-NEO1: An Oral Cancer Vaccine
NECVAX-NEO1 is based on a clinically validated plug & play platform technology for patient-convenient oral administration which is self-adjuvanted through the bacterial carrier. The turnaround time is short due to the straight-forward small scale manufacturing process, enabling treatment of patients with metastatic disease. The technology enables targeting of a large number of neoantigens of any size and is applicable to personalized as well as shared neoantigen-targeting approaches. The antigen epitopes are presented on the dendritic cell surface via MHC class I molecules that activates antigen specific CD8+ T cells. These activated T cells then initiate the cell death of the cells expressing the target antigens, thereby contributing to the immune response.
TG4050: A Viral Vectored Cancer Vaccine
TG4050 is developed in collaboration with a French biotech Transgene. Transgene platforms use an MVA which has a long track record of clinical safety and efficacy. Target antigens identified using AI are cloned into the virus genome and expressed in the patient upon delivery. The association of cancer target and a non-pathogenic virus leverage the natural sensitivity of the immune system to virus to induce a strong response against cancer neoantigens. The size of the genomic payload of the virus allows the selection of a large panel of cancer target, hence decreasing the risk of resistance and addressing the complexity of tumor heterogeneity.
ICT Platform
In addition to our vaccine design technology, NEC is conducting a wide range of research and development, including networks and cyber security, that will contribute to the manufacture and delivery of personalized vaccines.
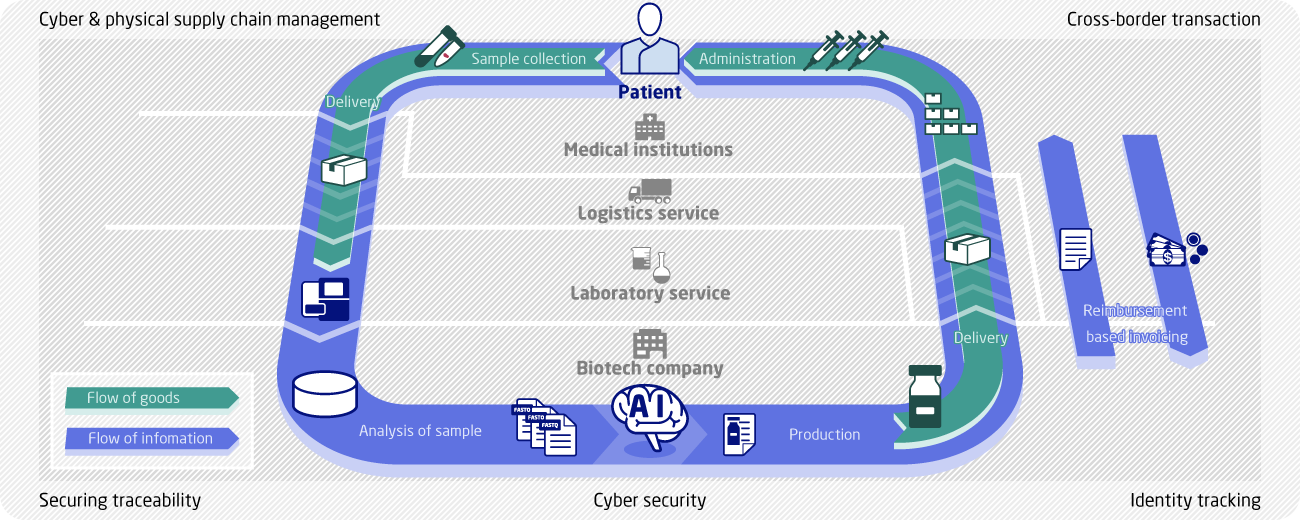
Securing Traceability
One very important aspect of personalized cancer vaccines is to ensure that vaccines based on information obtained from a given patient are administered to that same patient by ensuring a “chain of identity.” Strict logistical controls need to be introduced at key steps, such as the transfer of biological samples collected from patients and the transfer of vaccines. The logistical flow can be managed to a higher level by using biometrics to record and authenticate third parties involved in product handling. Another important aspect is the need for a high level of traceability in the exchange of data and the maintenance of data trails. Consequently, in addition to logistics management, the authenticity, integrity, and traceability of data exchanges must be securely maintained for a long period of time.
Acquisition and Storage of Genomic Data
Another important challenge is the protection and storage of a large amount of genomic data. This sensitive patient data calls for strict management and measures to protect against data exposure.
Manufacturing and Quality Assurance
Manufacturing operations and testing processes are automated to reduce turn-around-time (TAT) in the manufacturing process. In particular, the automation of quality-related tests is a useful means of improving quality control TAT while maintaining high quality standards, and we are carefully exploring the viability of AI technology for these applications.
For more details and other key considerations, see our white paper.
 White Paper
White Paper